Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji
Bunri Gijutsu, 52(2), p.103 - 107, 2022/03
We develop all-inclusive partitioning method for actinides and fission products in high-level radioactive waste. This process is based on the sequential solvent extraction. In order to recover Cs and Sr for the management by interim storage, crown ether compounds are employed. For the removal of Pd and Mo due to production of a stable vitrified object, methylimino-dioctylacetamide (MIDOA) is taken as an extractant. DGA can extract both actinides and trivalent lanthanides. In order to separate each other, dietylenetriamine-triacetic-diamide (DTBA) for the stripping reagent of MA. For the mutual separation of Am/Cm, DGA and DOODA extraction system is taken into consideration.
Sasaki, Yuji; Nakase, Masahiko*
Petorotekku, 43(11), p.782 - 787, 2020/11
As analog compounds of DGA (diglycolamide), MIDOA(methylimino-diacetamide) and TDGA(thia-diglycolammide) are used for the extractants of platinum group metals. These extractants can be extracted noble metals and oxyanions, which followed by HSAB theory. The high concentration of these metals can be also extracted by these compounds. The research of metal-complex structures gives the information on the ability and role for complex-formation, which will be useful for the development of novel extractants.
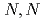 -dioctylacetamide and direct electrodeposition from loaded organic phase
-dioctylacetamide and direct electrodeposition from loaded organic phaseMatsumiya, Masahiko*; Song, Y.*; Tsuchida, Yusuke*; Sasaki, Yuji
Separation and Purification Technology, 234, p.115841_1 - 115841_8, 2020/03
Times Cited Count:17 Percentile:62.19(Engineering, Chemical)The development of solvent extraction and direct electrodeposition processes is an important task to reduce the volume of secondary wastes. In this study, the extraction of Pd(II) from hydrochloric/chloride media using methylimino-bis-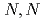 -dioctylacetamide (MIDOA) in three diluents (acetophenone; AP, 1,2-dichloroethane; DCE, or 1-octanol; OC) and the electrochemical behavior of the extracted Pd(II) complex in the MIDOA/AP bath was investigated. Pd(II) was found to be reduced to Pd(0) metal via a two-electron transfer between -2.38 V and -3.40 V. The potentiostatic electrodeposition of the extracted Pd(II) complex enabled us to recover the blackish electrodeposits, which were identified as Pd metal.
-dioctylacetamide (MIDOA) in three diluents (acetophenone; AP, 1,2-dichloroethane; DCE, or 1-octanol; OC) and the electrochemical behavior of the extracted Pd(II) complex in the MIDOA/AP bath was investigated. Pd(II) was found to be reduced to Pd(0) metal via a two-electron transfer between -2.38 V and -3.40 V. The potentiostatic electrodeposition of the extracted Pd(II) complex enabled us to recover the blackish electrodeposits, which were identified as Pd metal.
Sasaki, Yuji; Saeki, Morihisa*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 26(1), p.21 - 34, 2019/06
Times Cited Count:6 Percentile:26.61(Chemistry, Multidisciplinary)Three tridentate extractants and three masking reagents including O, N, and S donors have been developed and their properties are compared and discussed. The extractants are termed as tetraoctyl-diglycolamide (TODGA), methylimino-dioctylacetamide (MIDOA) and tetraoctyl-thiodiglycolamide (TDGA(C8)) and masking agents have the same central frame but with short alkyl chain. The results of the present study indicate that TODGA can extract mainly hard acid metals belonging groups 2-4,13-15 in periodic table, MIDOA can extract soft acid metals and oxyanions (groups 5-10, 16), and TDGA can extract soft acid metals (groups 10-11). Some spectrophotometric studies (UV, IR, and NMR) indicate the stoichiometry and effect of donor atoms for metal-complexation. The Hf values, the heat generation during complex formation, obtained by chemical calculation by DFT theory show the reverse-correlation with their extraction ability.
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo*; Yoshizuka, Kazuharu*
Proceedings of 21st International Solvent Extraction Conference (ISEC 2017) (Internet), p.131 - 134, 2017/11
Three tridentate extractants including soft and hard donor has been developed and examined. The extractants are termed as 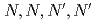 -tetraoctyl-diglycolamide (TODGA), methylimino-
-tetraoctyl-diglycolamide (TODGA), methylimino-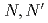 -dioctylacetamide (MIDOA) and
-dioctylacetamide (MIDOA) and 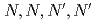 -tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
-tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
Sasaki, Yuji; Morita, Keisuke; Ito, Keisuke; Suzuki, Shinichi; Shiwaku, Hideaki; Takahashi, Yuya*; Kaneko, Masaaki*; Omori, Takashi*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference (GLOBAL 2017) (USB Flash Drive), 4 Pages, 2017/09
no abstracts in English
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo; Yoshizuka, Kazuharu*
Hydrometallurgy, 169, p.576 - 584, 2017/05
Times Cited Count:14 Percentile:56.4(Metallurgy & Metallurgical Engineering)The novel tridentate extractant including soft donor has developed and examined. The extractant, tetraoctyl-thiodiglycolamide (TDGA), is analogous structure to tetraoctyl-diglycolamide (TODGA) and methylimino-dioctylacetamide (MIDOA), but with sulfur donor instead of ether oxygen or nitrogen atoms of TODGA or MIDOA. From the present work, TDGA can extract silver, palladium, gold, and mercury from acidic solutions to n-dodecane. In addition to these results, the distribution ratios of hard and soft acid metals by using TDGA, TODGA, and MIDOA are compared, where the metal-complexations with each donor atom are investigated. 1H-NMR and IR studies for the metal-TDGA complexes indicate the role on donor atoms, S and N, of TDGA.
Sasaki, Yuji; Yoshimitsu, Ryo*; Nishihama, Shohei*; Shimbori, Yuma*; Shiroishi, Hidenobu*
Separation Science and Technology, 52(7), p.1186 - 1192, 2017/03
Times Cited Count:2 Percentile:9.15(Chemistry, Multidisciplinary)The new extractant, biuret(C8), is synthesized and tested for the solvent extraction of hard acid metals like actinides and soft acid metals. This compound has the similar central frame to malonamide but with 2-NH introduced into the central frame, then both amidic oxygen and nitrogen atoms may bond with metals. From the present work, not only hard acid metals, but also soft acid metals can be extracted by biuret(C8) from nitric or perchloric acids to n-dodecane. The extractability for biuret(C8) is compared with other representative extractants, malonamide, TODGA and MIDOA. It is clear that the distribution ratio(D) of U and Pu by biuret(C8) is similar to those for malonamide, but with lower values than those for TODGA and MIDOA, and D for soft acid metals and oxonium anions show higher values than those for TODGA and malonamide and lower than those for MIDOA.
Sasaki, Yuji; Morita, Keisuke; Shimazaki, Shoma*; Tsubata, Yasuhiro; Ozawa, Masaki*
Solvent Extraction Research and Development, Japan, 23(2), p.161 - 174, 2016/05
We examined the masking effects of 16 water-miscible reagents, on the extraction of Mo, Re, Ru, and Pd. The extractants, methylimino-dioctylacetamide (MIDOA) and hexaoctyl-nitrilotriacetamide (NTAamide(C8)), show significantly high distribution ratios for these metals, were employed in this study. Masking effects were observed as a decrease of distribution ratio with an increase of masking agent concentration in these extraction systems. The results showed that Pd and Ru can be masked by similar reagents including N- or S- donor atoms, which suppressed the extraction into the organic phase. In contrast, distribution ratio of Mo was only slightly masked by the above mentioned reagents. The masking of Mo was achieved using complexing agents having a central N(CH C(P)=O)
C(P)=O) framework that is important for this purpose. A masking agent for Re was not found in this study.
framework that is important for this purpose. A masking agent for Re was not found in this study.
Sasaki, Yuji; Suzuki, Shinichi; Morita, Keisuke
no journal, ,
Many kinds of metals with high concentration exist in high level radioactive waste (HLW). Most of metals have high radioactivity and strong heat, then there should be removed from aqueous phase. Target metals in HLW are as follows, actinides, lanthanides, zirconium, molybdenum, technetium, cesium, strontium, and palladium, due to long range radioactivity, huge environmental burden, and high concentration then troublemsome metals. We try to separate these metals by solvent extraction. Actinides and lanthanides can be extracted by TODGA (tetraoctyl-diglycolamide) from nitric acid, Zr is done by malonamide (or HDEHP), Mo, Re (substitution for Tc) and Pd can be extracted by MIDOA (methylimino-dioctylacetamide), and Sr and Cs can be removed by crown ether compounds. These extractants, except for HDEHP, belong to CHON compounds, which leads less secondary waste generation.
Sasaki, Yuji
no journal, ,
Since initially reported TODGA extractant at 2001, I have developed four analogous extractants, which have O, S, and N donor at ether position. The distribution ratios of over 70 metals using these extractants were obtained in order to know the all-inclusive extractability. Concerning with this, I developed water-soluble reagents with the same central frame but with short alkyl chains and examined their masking effects.